Introduction
The world believed that all chemical reactions were irreversible until
1803 when French chemist Claude Louis Berthollet introduced the concept
of reversible reactions.
Irreversible Reactions
As children, we learned that chemical
reactions occurred when reactants reacted with each other to form
products. These unidirectional reactions are known as irreversible
reactions. In other words, irreversible reactions are reactions where
the reactants convert to products and where the products cannot convert
back to the reactants. These reactions are essentially like cooking eggs. Cooking the eggs is an example of irreversible reaction,It does not matter how we cook the eggs,when we have cooked eggs we can not bring it back to uncooked postion
As stated above, a egg cannot be "uncooked" and so the reaction is irreversible.
A real-life example of an irreversible
reaction is combustion. Combustion usually involves the burning of an
organic compound, like a hydrocarbon, and oxygen to produce carbon
dioxide and water. Since water is stable in its polyatomic state, like
below, it will not react with the other product, CO2, to form the reactants. Combustion can take the following form:
CxHy + O2 → CO2 + H2O
Reversible Reactions
In reversible reactions, the reactants and products are never used up. In fact, they are both constantly reacting and being produced. A reversible reaction can take the following summarized form:
his reversible reaction can be broken into two reactions.
Reaction 1:
Reaction 2:
These two reactions are occurring simultaneously, which means that the reactants are reacting to yield the products, as the products are reacting to produce the reactants.
Collisions of the reacting molecules cause chemical reactions in a
closed system. After products are formed, the bonds between these
products are broken because the molecules collide with each other,
producing sufficient energy needed to break the bonds of the product and
reactant molecules.
Below is an example of the summarized form of a reversible reaction and a breakdown of the reversible reaction N2O4 ↔ 2NO2
Reaction 1 and Reaction 2 happen at the same time because they are in a closed system.
Blue: Nitrogen Red: Oxygen
Reaction 1
Reaction 2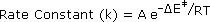
Imagine a ballroom. Let reactant A be 10
girls and reactant B be 10 boys. As each girl and boy goes to the dance
floor, they pair up to become a product. Once five girls and five boys
are on the dance floor, one of the five pairs breaks up and moves to the
sidelines, becoming reactants again. As this pair leaves the dance
floor, another boy and girl on the sidelines pair up to form a product
once more. This process continues over and over again, representing a
reversible reaction.
Unlike irreversible reactions,
reversible reactions lead to equilibrium because reversible reactions
have the reaction proceeding in both directions while irreversible
reactions only have the reaction proceeding in one direction.
If the reactants are being made at the same rate as the products are
being made, a dynamic equilibrium exists. For example, if a water tank
is being filled with water at the same rate as water is leaving the tank
(through a hypothetical hole), the amount of water remaining in the
tank remains consistent.



0 comments:
Post a Comment